Describe a Bond Within a Polar Molecule
This means that the bonding electrons usually spend more time around the atom that attracts the shared electrons the strongest most electronegative atom than they do around the atom that attracts the shared electrons the weakest least electronegative atom. The polarity of water molecules means that molecules of water will stick to each other.
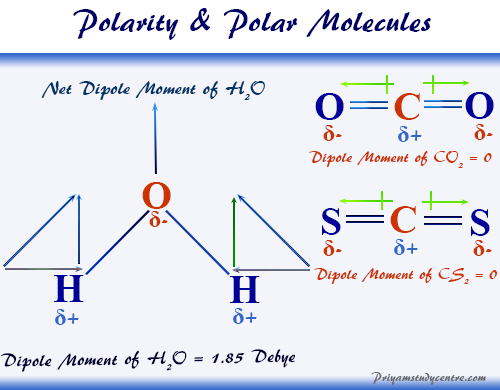
Polarity Of Bonds Polar Molecules Definition And Examples
2 A molecule can have polar bonds but be non-polar overall if the bond polarities cancel out.

. A bond is polar when the two atoms that are participating in the bond formation have different electronegativities. I assume you are referring to the water molecule H_2O. And hydrogen bonding occurs when hydrogen is bound to a strongly electronegative element such as oxygen or nitrogen or fluorine.
In polar molecule all the bonds collectively should produce a polarity. The greater the dipole moment of a molecule the more polar its bonds. It results from having polar bonds and also a molecular structure where the bond polarities do not cancel.
The property of cohesion describes the ability of water molecules to be attracted to other water molecules which allows water to be a sticky liquid. The oxygen atom has two lone pairs. A polar molecule is one where the charge distribution around the molecule is not symmetric.
A polar molecule is formed when there is asymmetrical distribution of charges that is the electron density is greater on one side and lesser on. For example water has polar bonds and its bent geometry means that. Water H 2 O is polar because of the bent shape of the molecule.
Weak chemical bond formed by the attraction of positively charged hydrogen atoms to other negatively charged atoms diffusion The process by which molecules move from an area of higher concentration to an area of lower concentration. A polar molecule is characterized by the uneven distribution of the electrons that form the covalent bonds between each atom in the molecule resulting in a slightly positively charged side and a slightly negatively charged side. In the case of water hydrogen.
Why are the bonds in a water molecule polar covalent and how does that affect the interactions between water molecules. A polar bond is one where the charge distribution between the two atoms in the bond is unequal. The molecule is bent.
H_2O is indeed a polar molecule because it has polar bonds between the oxygen atom and the two hydrogen atoms and because its molecular geometry allows for the two bonds dipole moments to add to each other instead of cancelling out. Well the intramolecular bond the bonds within the molecule are simply covalent H-O bonds. Each O-H bond is polar.
It is a polar molecule and is highly associated because of strong intermolecular hydrogen bonding. Polar bonds occur when the charge distribution between two atoms is unequal. For example if two atoms do not share electrons equally it is possible their bonds are polar.
The molecule is asymmetrical. This is due to the shape of the molecule for example CO 2 has two polar bonds and a linear shape the bond polarities. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
The two hydrogen atoms and one oxygen atom within water molecules H 2 O form polar covalent bonds. Polarity of a Water Molecule. Polar bonds are caused by unequal sharing of electrons in a bond due to a difference in electronegativity of 05 units or greater between the atoms in the bond.
This is called hydrogen bonding. Hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly positive hydrogen ions and other slightly negative ions. This occurs because of the differences in electronegativity between atoms of different elements.
What is the difference between Polar Bonds and Polar Molecules. Since oxygen is more electronegative than hydrogen the. Oxygen is more electronegative than hydrogen.
Describe what occurs in and results from a molecule being polar. Polar molecules possess polar bond. The ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
But the intermolecular bonds the bonds BETWEEN water molecules are the result of hydrogen bonding. Each hydrogen atom is bonded to the oxygen atom with a single bond. Polarity makes water a good solvent gives it the ability to stick to itself cohesion stick to other substances adhesion and have surface tension due to.
This is an example of polar covalent chemical bonding. Describe how nonpolar covalent bonds are formed. However if you have to describe the ion you can use the phrase the like a polar molecule because I3- is soluble in water.
A polar molecule is one where the charge distribution is also asymmetric so there are partial charges on the molecule. A bond is polar when an atom is more electronegative than the other. When solutes are added to water they may.
Describe how polar covalent bonds are formed. Some bonds or molecules are polar when electrons are shared unequally. Water or dihydrogen monoxide H2O is a polar molecule because of polar covalent bonds between two hydrogens and one oxygen.
The asymmetry and the polar bonds produce an overall molecular dipole. A bond is polar when on atom is more electronegative than the other or the molecule is asymmetrical in geometry. Covalent Bonds How do covalent bonds Covalent bonds form between atoms of similar.

Science Coverage Is Hcl Polar Or Nonpolar Covalent Bonding Molecular Geometry Molecules

Polar Covalent Bond Definition And Examples
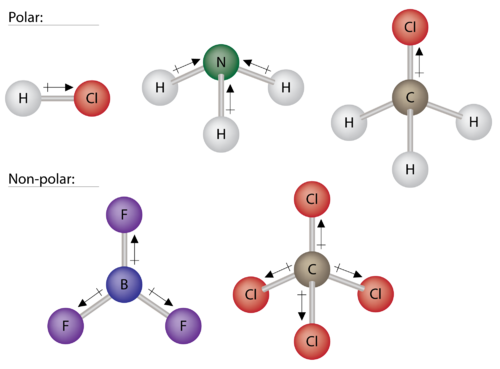
Polar Molecules Chemistry For Non Majors

How Do Molecular Compounds Bond Example Hydrogen Bond Molecular Bond

3 10 Water Is The Highest Cohesive Of The Non Metallic Liquids Cohesion Embraces Hydrogen Bonds Together To Chemistry Chemistry Classroom Teaching Chemistry

Polar And Non Polar Covalent Molecules Polar Vs Nonpolar Youtube Playlist Science Chemistry Chemistry Science Activities

Polar And Nonpolar Covalent Bonds Definitions Molecules And Examples

Chemistry Intermolecular Forces Polar Bonds And Polarity Teaching Chemistry Chemistry Classroom Physics Lessons
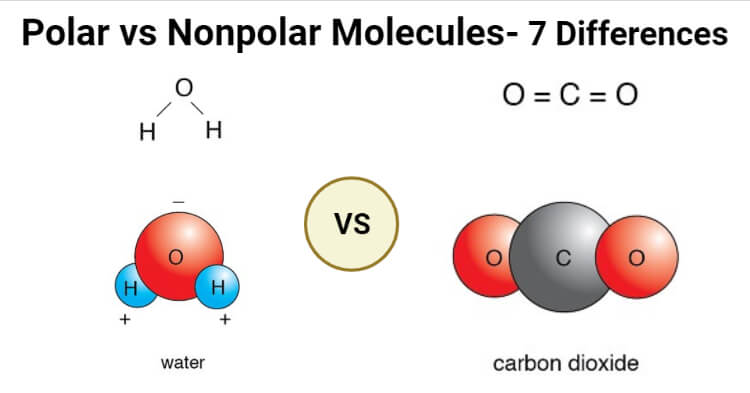
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples
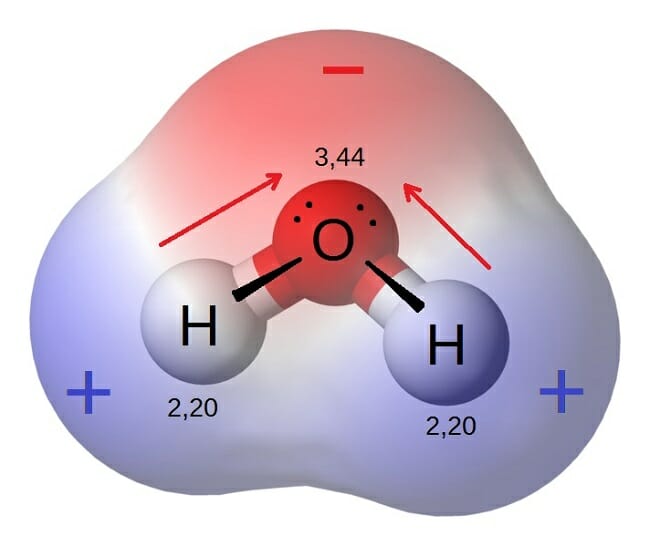
Polar Molecule Definition And Examples Biology Dictionary

We Are Going To Look At Polar Covalent Now What Is Polar Covalent Bond Polar Covalent Is A Description Of A Bond Th Covalent Bonding Bond Chemical
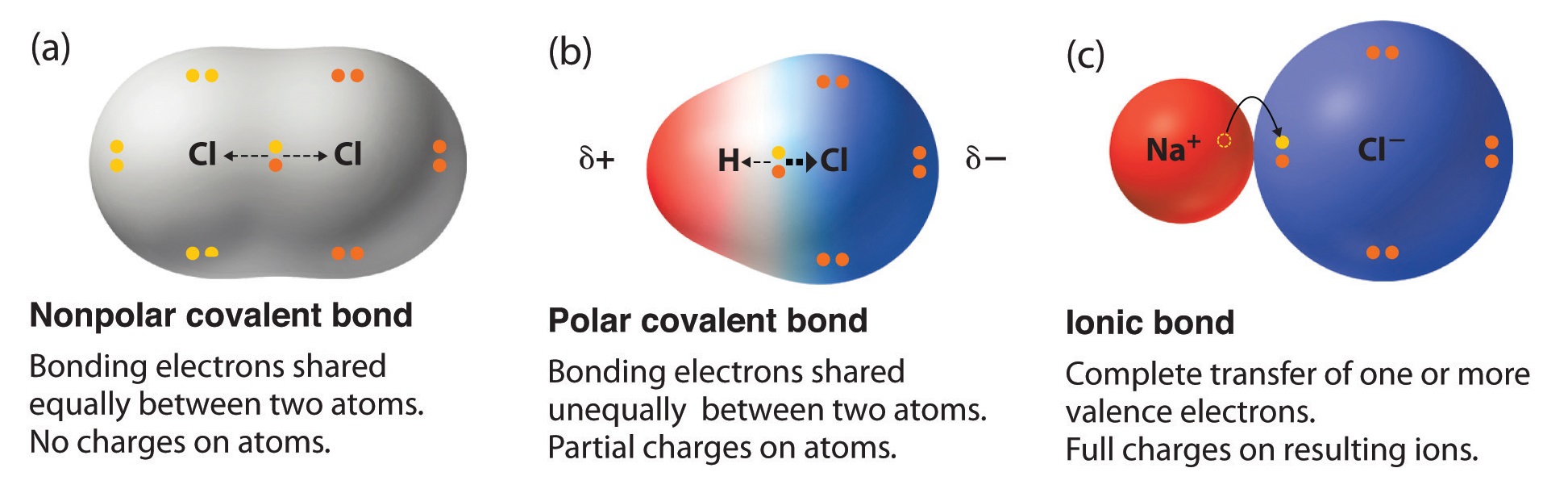
8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts

Polar Molecules Chemistry For Non Majors

Image Result For Polar Vs Nonpolar Molecules Covalent Bonding Chemical Bond Study Chemistry

Polar Nonpolar Covalent Bonds Ch 6 Youtubea Little Too Detailed Though Covalent Bonding Chemistry Classroom Chemistry Lessons

Examples Of Polar And Nonpolar Molecules Chemical Bond Chemistry Covalent Bonding


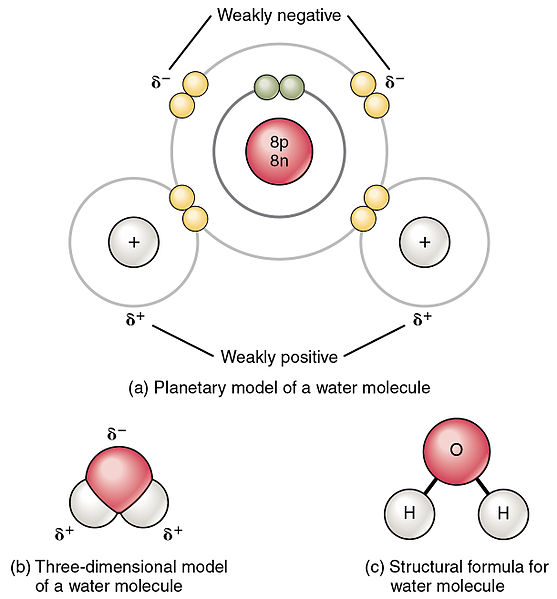
Comments
Post a Comment